
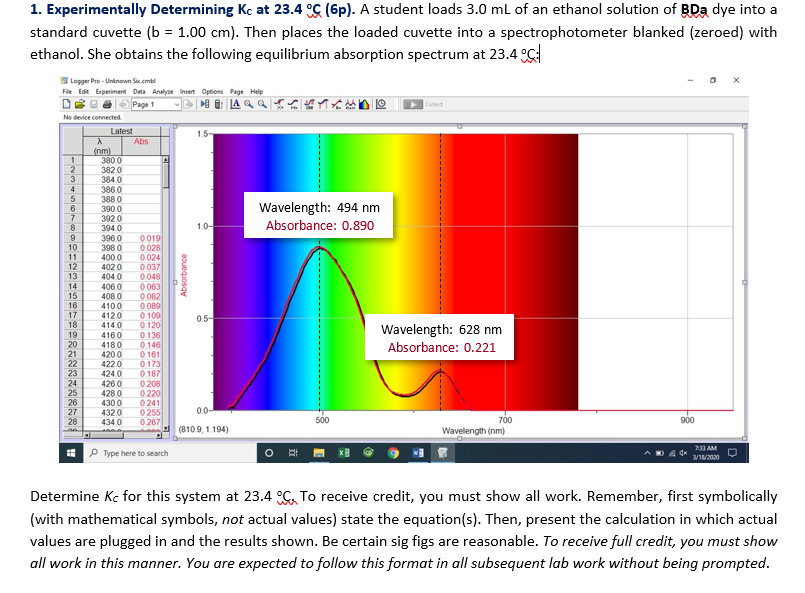
0.5 g/L bromophenol blue (Na 2 Bp = Na 2 C 19 O 5 Br 4 SH 8 FW 669.96 g/mol).Collect waste in waste hood to be neutralized.Be sure to wear gloves when handling bromophenol blue.** Bromophenol blue solution may stain your skin. Please rinse affected area with soap and water. ** 4M sodium hydroxide is corrosive and an irritant to eyes and skin. Please rinse affected area with copious amounts of water. Should we measure our solutions at 590nm? 610nm? 570nm? If we want our initial absorption readings to be less than 1.5, what wavelength should we use in lab?įigure 1: Absorption spectrum of 0.03 g/L Bromophenol blue solution Experiment To print instructions, select the portion that you wish to print, right click and choose "print" to prevent printing the entire web page. Notice that the maximum absorption is much higher than ideal for our spectrophotometers to give us an accurate measurement. You will need to use the spectrum below in order to determine the wavelength at which to set your spectrophotometers. This permits evaluation of the overall rate law and the rate constant. By monitoring the concentration of Bp 2- in two or more experiments with different concentrations of excess hydroxide, one can determine the order with respect to the also. Since Bp 2- is colored, its concentration can easily followed spectrophotometrically. In determining the rate law for this reaction, the hydroxide ion should be in large excess so that only the concentration of Bp 2- decreases during any one experiment. In a bimolecular reaction, such as the one above, determining the reaction order with respect to one species can be simplified by choosing reaction conditions in which the concentration of one reactant is so large that it is essentially constant during the course of the reaction. Here, the slope is equal to the rate constant. If the reaction is second order with respect to X, then a plot of 1/ vs. time is linear and the slope of the line is the negative of the rate constant. If a reaction is first order with respect to a particular species, X, then a plot of ln vs. One way to determine the order of a chemical reaction is to monitor the concentration of the reactant(s) versus time. To determine the rate law, the unknowns k, m, and n must be found. Where k is the overall rate constant, and are the time-dependent concentrations of the bromophenol blue dianion and the hydroxide ion, respectively, m is the order of the reaction with respect to Bp 2-, and n the order with respect to OH. The overall rate law that describes this reaction can be written as follows: rate = k m n The disappearance of the blue dianion in an excess of hydroxide proceeds at a rate that is easy to monitor as the half-life is on the order of minutes. The blue dianion, Bp 2-, in this aqueous solution reacts with excess hydroxide ion to form a colorless tri-negative ion Bp(OH) 3- as shown below in equation 1. In this lab, you will be supplied with an aqueous solution of Na 2Bp. Better understand rate laws, rate constants, and orders of reaction.īromophenol blue, H 2Bp, is an organic compound having two acidic protons (H +'s) which are readily abstracted to form a blue dianion, Bp 2.Determine the rate law for the fading of bromophenol blue in basic solution.They were not prepared, however, for what they saw on the next page. They answered the question and turned the page. "Cool" they thought." This is gonna be easy". It was a simple question involving molarity calculations. They looked at the first problem which was worth 5 points. The next day the professor placed them in separate rooms and handed them the test booklet. The professor thought it over and told them they could make up the final the next day. They didn't have a spare and couldn't get help for a long time, that's why they were late in getting to campus. They told him that they "had a flat tire" on their way to school. After the final, they met with the professor to explain why they were late. They had a great time, however, they were hung over the next day and didn't make it to the final on time. They were so confident that the weekend before finals they decided to go out and party. They did pretty well on all the quizzes, midterms and exams and had a solid "A" going into the final. Jokes Q: Why are chemists great for solving problems?


 0 kommentar(er)
0 kommentar(er)
